A) A only
B) B only
C) C only
D) both B and C
E) A, B, and C
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate the line-angle structure that corresponds to the condensed structure, HOCH2C(O) CH(CH3) 2.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Stabilization of a charged species usually results when this species can be more accurately depicted as a hybrid of several resonance forms. Why is this the case?
Correct Answer

verified
Stabilization result...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Calculate the molecular formula for the organic compound whose quantitative elemental analysis showed 48.6% caron and 8.1% hydrogen by weight.
A) CH2O
B) C2H4O2
C) C2H6
D) C3H6O
E) C3H6O2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The element with the electronic configuration 1s22s22p63s1 is ________.
Correct Answer

verified
Correct Answer
verified
Essay
One resonance structure of a cation is shown. Provide the other reasonable resonance structures. 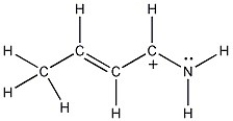
Correct Answer

verified
Correct Answer
verified
Essay
Provide the line-angle formula (skeletal structure) for (CH3CH2)2C=O.
Correct Answer

verified
Correct Answer
verified
True/False
When filling two or more orbitals of the same energy with electrons, the electrons will go into different orbitals rather than pair up in the same orbital.
B) False
Correct Answer

verified
Correct Answer
verified
Essay
Draw the structure of the conjugate acid of acetone 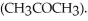
Correct Answer

verified
Correct Answer
verified
Essay
The compound phenol is shown below. Provide the structure of the conjugate base of phenol. 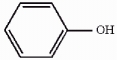
Correct Answer

verified
Correct Answer
verified
Essay
Provide the products of the following acid-base reaction. (CH3)3NH+ + HO-→
Correct Answer

verified
Correct Answer
verified
Essay
Add the appropriate formal charge to each atom in the molecule below. It is not necessary to indicate formal charges when zero. (All non-bonding electrons are included.) 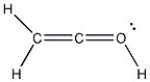
Correct Answer

verified
Correct Answer
verified
Short Answer
Covalent bonds may be polar or nonpolar. What property of the atoms forming a given bond determines this?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures (a-d) is another resonance structure of the following organic molecule? 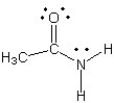
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for boric acid, B(OH)3, including all non-bonding lone pairs.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for 2-propanol, CH3CH(OH)CH3, including all non-bonding lone pairs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The conjugate acid of ammonia, NH3, is ________.
A) NH2-
B) NH2OH
C) NH4+
D) none of the above
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of a 150 mL aqueous solution of 2.13 x 10-3 M HCl is ________.
A) -3.000
B) 3.000
C) 2.672
D) 2.130
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following condensed formulas represents the same compound as the line-angle structure shown? 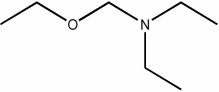
A) CH3CH2CH2OCH2CH2CH2N(CH2CH2CH3) 2
B) CH3CH2CH2OCH2N(CH2CH3) 2
C) CH3CH2OCH2N(CH2CH3) 2
D) CH3CH2OCH2N(CH2CH2CH3) 2
E) CH3ON(CH3) 2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the molecular formula for the molecule shown? 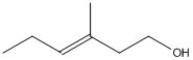
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 127
Related Exams