A) The molecule must be cyclic.
B) The molecule must be planar.
C) The molecule must be completely conjugated.
D) The molecule must have 4n pi electrons.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct assignment of the names of the following substituted benzenes? 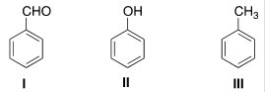
A) I = Phenol; II = anisole; III = toluene
B) I = Benzaldehyde; II = phenol; III = anisole
C) I = Benzaldehyde; II = phenol; III = toluene
D) I = Phenol; II = anisole; III = styrene
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a criterion for antiaromaticity?
A) The molecule must be cyclic.
B) The molecule must have (4n + 2) pi electrons.
C) The molecule must be planar.
D) The molecule must be completely conjugated.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ions is aromatic? 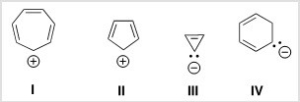
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the structure of benzene is not true?
A) Benzene is planar.
B) Benzene has three short double bonds alternating with three longer single bonds.
C) The electrons in the pi bonds are delocalized around the ring.
D) Benzene has six pi electrons.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Where do the protons in benzene appear in the 1H NMR spectrum?
A) Around 1600 cm-1
B) Around 120 ppm
C) Around 0 ppm
D) Around 7 ppm
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 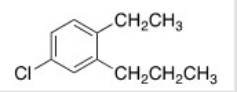
A) 1-Chloro-4-ethyl-3-propylbenzene
B) 1-Chloro-4-ethyl-5-propylbenzene
C) 4-Chloro-1-ethyl-2-propylbenzene
D) 4-Chloro-2-propyltoluene
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What orbitals are used to form the indicated bond? 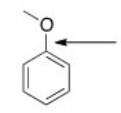
A) sp3
B) sp2
C) p
D) sp3 and sp2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the following compound not aromatic? 
A) It has 4n electrons.
B) It is not cyclic.
C) It has 4n+2 electrons.
D) The pi electron system is not continuous.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What orbitals are used to form the indicated bond? 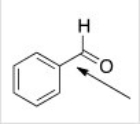
A) sp3
B) sp2
C) p
D) sp3 and sp2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following heterocycles is not aromatic? 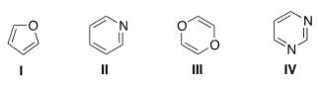
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 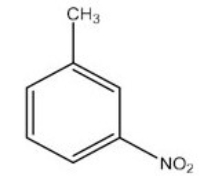
A) o-nitrotoluene
B) m-nitrotoluene
C) p-nitrotoluene
D) 3-nitroanisole
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 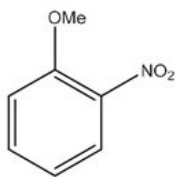
A) o-nitroanisole
B) m-nitroanisole
C) p-nitroanisole
D) 2-nitroaniline
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct assignment of the names of the following substituted benzenes? 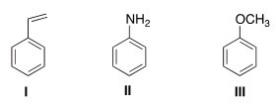
A) I = Styrene; II = aniline; III = anisole
B) I = Toluene; II = anisole; III = styrene
C) I = Styrene; II = anisole; III = phenol
D) I = Toluene; II = aniline; III = anisole
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct structure for toluene? 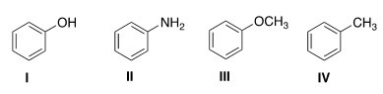
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct assignment of the names of the following fused aromatic compounds? ![What is the correct assignment of the names of the following fused aromatic compounds? A) I = naphthalene; II = anthracene; III = phenanthrene B) I = naphthalene; II = phenanthrene; III = anthracene C) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene D) I = anthracene; II = naphthalene; III = phenanthrene](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7c0_c990_a9af_d5fba3396182_TB7662_00.jpg)
A) I = naphthalene; II = anthracene; III = phenanthrene
B) I = naphthalene; II = phenanthrene; III = anthracene
C) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene
D) I = anthracene; II = naphthalene; III = phenanthrene
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is aromatic? 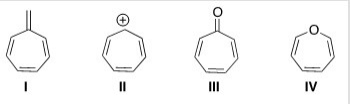
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct structure for 1-bromo-2,4-dimethoxybenzene? 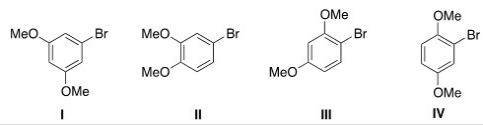
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the structure of a compound of molecular formula C10H14O2 that shows a strong IR absorption at 3150 ¾2850 cm-1 and gives the following 1H NMR absorptions: 1.4 (triplet,6H) ; 4.0 (quartet,4H) ; and 6.8 (singlet,4H) ppm? 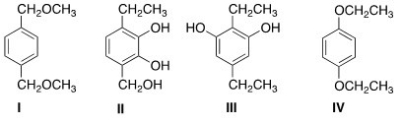
A) I
B) II
C) III
D) IV
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the following compound not aromatic? 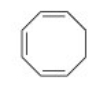
A) It has 4n pi electrons.
B) It is not cyclic.
C) It has 4n+2 pi electrons.
D) The pi electron system is not continuous.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 47
Related Exams