A) alphabetical order
B) order of increasing neutron content
C) reverse alphabetical order
D) order of increasing metallic properties
E) order of increasing atomic number
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the ionic compound KBrO4 is _ .
A) potassium perbromite
B) potassium bromide
C) potassium hypobromate
D) potassium bromate
E) potassium perbromate
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of elements below should be the most similar in chemical properties?
A) I and Br
B) Cs and He
C) B and As
D) C and O
E) K and Kr
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element X has two naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. 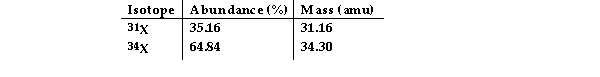
A) 34.02
B) 30.20
C) 35.22
D) 32.73
E) 33.19
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The charge on the manganese in the salt MnF3 is _ .
A) - 2
B) - 1
C) +1
D) +3
E) +2
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
the possible oxication numbers for gold are 1+ and 2+.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the symbol below, x = . X C 6
A) 6
B) 7
C) 19
D) 13
E) not enough information to determine
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the empirical formula of the ionic compound that forms from magnesium and oxygen.
A) MgO2
B) MgO
C) Mg2O2
D) Mg3O2
E) Mg2O
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is chromium(III) oxide?
A) Cr3O
B) Cr2O4
C) Cr2O3
D) Cr3O2
E) CrO3
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following species has as many electrons as it has neutrons?
A) . 14C
B) . 40Ca2+
C) . 1H
D) . 14C2+
E) . 19F-
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Methane and ethane are both made up of carbon and hydrogen. In methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a ratio of 3: 1 by mass. In ethane, there are 24.0 g of carbon for every 6.00 g of hydrogen, a ratio of 4: 1 by mass. This is a statement of the law of _.
A) multiple proportions
B) conservation of matter
C) octaves
D) conservation of mass
E) constant composition
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of PCl3 is _ .
A) trichloro potassium
B) potassium chloride
C) phosphorous(III) chloride
D) phosphorus trichloride
E) monophosphorous trichloride
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following selected postulates of Dalton's atomic theory: i. Each element is composed of extremely small particles called atoms. ii. Atoms are indivisible. iii. Atoms of a given element are identical. iv. Atoms of different elements are different and have different properties. Which of the postulates is(are) no longer valid?
A) (iii) and (iv)
B) (i) and (ii)
C) (ii) only
D) (iii) only
E) (ii) and (iii)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur forms an ion with a charge of _ .
A) - 2
B) +2
C) +3
D) - 6
E) +6
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The most metallic halogen is astatine.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element forms an ion with the same charge as the ammonium ion?
A) nitrogen
B) oxygen
C) potassium
D) chlorine
E) calcium
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, the smallest and lightest subatomic particle is the .
A) electron
B) proton
C) nucleus
D) alpha particle
E) neutron
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of the most common isotope of gold, 197Au, has protons, _ _ neutrons, and electrons.
A) 79, 118, 79
B) 79, 118, 118
C) 79, 197, 197
D) 197, 79, 118
E) 118, 79, 39
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the choices below, which one is not an ionic compound?
A) PbCl2
B) PCl5
C) MoCl6
D) NaCl
E) RbCl
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species below is the nitrate ion?
A) NH4+
B) N3-
C) NO3-
D) NO2-
E) N3-
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 161 - 180 of 201
Related Exams