A) soot
B) ozone
C) sulfur dioxide
D) carbon monoxide
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Would a woman that inhaled 15,000 liters of air per day exceed the 24-hr average for inhalation of SO2 if she were exposed to 1150 μg of SO2 each day?
Correct Answer

verified
NO
Explanation: Consult Table ...View Answer
Show Answer
Correct Answer
verified
Explanation: Consult Table ...
View Answer
Multiple Choice
There are approximately 2 × 1022 molecules and atoms in each breath we take and the concentration of CO in the air is approximately 9 parts per million.Approximately how many CO molecules are in each breath we take?
A) 2 × 1015
B) 1.8 × 1017
C) 2 × 1017
D) 2 × 1029
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When assessing the risk of an air pollutant,which does not play a role in considering someone's exposure to the pollutant?
A) a person's lung capacity
B) a person's breathing rate
C) the toxicity of the pollutant
D) the concentration in air of the pollutant
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which diagram(s) best represent(s) only diatomic molecules? 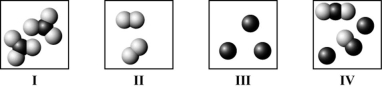
A) I only
B) II only
C) I and II only
D) II and IV only
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of five major gaseous components of air,which is the only one to vary significantly in concentration from place to place and from day to day?
A) water vapor
B) carbon dioxide
C) nitrogen
D) argon
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the proper coefficients for each substance to yield a balanced equation. 
A) 1,1,1
B) 2,1,1
C) 2,1,2
D) 2,1,1.
F) A) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which pollutant are you more likely to encounter in dangerous concentrations indoors rather than outdoors?
A) nitrogen dioxide
B) carbon monoxide
C) ozone
D) sulfur dioxide
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance this equation P4 + Cl2 → PCl5 with the smallest whole number coefficients.Choose the answer that is the sum of the coefficients.Do not forget coefficients of "one".
A) 7
B) 9
C) 11
D) 13
E) 15
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ozone is considered an air pollutant in the ________ but is a valuable protective layer in the __________.
A) troposphere;stratosphere
B) stratosphere;mesosphere
C) stratosphere;troposphere
D) mesosphere;stratosphere
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The quantity 8.7 × 105 g expressed in standard decimal notation is:
A) 0.000087 g
B) 870.000 g
C) 0.0000087 g
D) 870,000 g
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ozone is a secondary pollutant.A secondary pollutant is
A) not as hazardous as a primary pollutant.
B) not produced directly but as the product of the interaction of two or more pollutants.
C) one that is naturally present in our atmosphere.
D) one that is less hazardous than a primary pollutant.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Catalytic converters reduce the amount of ________ in car exhaust.
A) O3
B) CO2
C) CO
D) N2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The EPA limit for CO is 9 ppm.Express this number as a percentage.
A) 90%
B) 9%
C) 0.09%
D) 0.0009%
F) A) and D)
Correct Answer

verified
D
Correct Answer
verified
Short Answer
Which of the following are examples of technological advances that have reduced air pollution? __ Paint with reduced VOCs __ Catalytic converters __ Burning gasoline in leaf blowers __ Low sulfur Diesel fuels
Correct Answer

verified
X Paint with reduced VOCs
X C...View Answer
Show Answer
Correct Answer
verified
X C...
View Answer
Multiple Choice
Which substance is an element?
A) NO2
B) NaCl
C) N2
D) CH4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the compound formed by combining carbon atoms  With oxygen atoms
With oxygen atoms  To form
To form  Is
Is
A) carbon oxide.
B) monocarbon dioxide.
C) carbon dioxide.
D) carbonate.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which correctly pairs an indoor pollutant with its source?
A) formaldehyde and unvented space heaters
B) O3 and electrical arcing
C) radon and glues and solvents
D) nicotine and paint and paint thinners
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most numerous of the elements are the
A) metals.
B) non metals.
C) metalloids.
D) noble gases.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the balanced chemical equation showing hydrogen peroxide (H2O2) decomposing into hydrogen (H2) and oxygen (O2) ?
A) H2O2 → H2 + O2
B) H2 + O2 → H2O2
C) 2 H2 + O2 → 2 H2O2
D) 2 H2O2 → 2 H2 + O2
F) A) and C)
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 76
Related Exams