A) represents the total energy of radiation absorbed.
B) describes the effect of radiation on living humans.
C) is the amount of radiation necessary to treat food products.
D) accounts for the different properties of different types of radiation.
E) only applies to alpha emissions.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fusion is important
A) as a current method of producing electricity.
B) for understanding the formation of the elements.
C) for triggering fission bombs.
D) b and c.
E) as an energy source for space programs.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Strontium-90 decays by emission. Which of the following is the decay product?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fission power produces unstable nuclides which have varying half-lives. One common product is cesium, with several different nuclides being produced. One nuclide that is problematic is 137Cs which decays by emission with a half-life of 30.2 years. How many years will it take for 1.5 kg of 137Cs to decompose to only 12 g of 137Cs?(If needed, use the following equation: 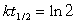
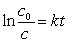 )
)
A) 60.4
B) 181.2
C) 210.4
D) 213.4
E) 240.6
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best explains why heavier elements don't form in first-generation stars?
A) The concentration of reactants is not high enough.
B) The heavier elements are only made in a supernova.
C) The spectrum of the star contains too much red.
D) Second generation stars are hotter.
E) There are free neutrons in a 1st generation star.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
238U is an unstable nuclide that undergoes multiple decay events. One sequence is - - - - . The product nuclide is
A) 220Rn.
B) 222Rd.
C) 218Po.
D) 222Rn.
E) 220Rd.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Neutron-capture reactions are
A) endothermic, and only occur at high temperature.
B) exothermic, as neutron is attracted by strong nuclear force.
C) exothermic, as they are spontaneous.
D) result in stable reaction products.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All waste from a nuclear fission reactor
A) are solid and composed of the daughter products of the fission reaction.
B) can be safely disposed of as they are composed of low grade radioactive materials.
C) include thermal waste in the form of warm water.
D) result from melt down of the reactor.
E) is radioactive and poses a potential threat to the environment.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radioactive waste nuclides with short half-lives are usually less of a problem for disposal because they are quite active but only for a short time. For example, 134 Cs has a half-life of 2.1 years. If initially there were 1.5 kg of 134Cs, how much of this nuclide would be present in 14.7 years?(If needed, use the following equation: 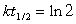
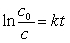 )
)
A) 1.2 kg
B) 120 g
C) 12 g
D) 10 g
E) 14.7 g
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nuclei that lie below the belt of stability are characterized by
A) high numbers of neutrons.
B) low neutron to proton ratio.
C) high neutron to proton ratio.
D) particle emission.
E) green colours.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the definition of critical mass?
A) mass needed to start a nuclear bomb
B) mass with a neutron recapture ratio of 1
C) mass with a neutron recapture ratio less than 1
D) mass needed to be detected by airport security
E) mass needed to start a nuclear reactor
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Seaborgium (Z= 106) can be synthesized by the bombardment of 249Cf with 16O. If four (4) neutrons are produced in the reaction, what is the mass number of the Sg nuclide?
A) 259
B) 260
C) 261
D) 262
E) 264
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is electricity made from the energy released at a nuclear power plant?
A) via hot water
B) via steam
C) via heavy water
D) via refrigeration
E) via cooling towers
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
222Rn is an unstable nucleus that is hazardous. Several pathways are known for its decomposition. One is - - - - - . The product nuclide is
A) 210Tl.
B) 210Pb.
C) 210Po.
D) 214Po.
E) 206Tl.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which force is responsible for repulsions in the nucleus?
A) gravitational force
B) electrical force
C) magnetic force
D) strong nuclear force
E) the star wars force
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What radioactive particle do you need to be concerned about that can go through clothing but not through your body?
A) alpha
B) beta positive
C) beta negative
D) gamma
E) neutron
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which nuclei would undergo neutron capture and subsequent decay to produce 99Tc, two particles and one particle?
A) 106Ag
B) 107Ag
C) 99Mo
D) 98Mo
E) 98Ru
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following factors limit the radio-dating techniques?
A) The half-life of the isotope.
B) The amount of background radiation.
C) The quantity of sample.
D) all of the above
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Typically how old can an artifact found in an archeological dig be and still allow you to use Carbon-14 dating to analyze its age? (14C, t1/2 = 5730 years.) (If needed, use the following equations: 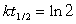
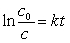 )
)
A) 1 half-life
B) 2 half-lives
C) 4 half-lives
D) 5 half-lives
E) can use it to determine the age of anything.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Seborgium's most stable isotope contains one-hundred-six protons and one-hundred-fifty-seven neutrons. This nuclide's symbol is
A) ![]() .S
.S
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 44
Related Exams