A) 10.0
B) -10.0
C) 4.0
D) -4.0
E) 1.0 × ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the Brønsted-Lowry definition,________.
A) an acid is a proton acceptor
B) a base produces ![]() ions in aqueous solutions
ions in aqueous solutions
C) a base is a proton donor
D) a base is a proton acceptor
E) an acid acts as the solvent
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an acid reacts with a metal like Al,the products are ________.
A) water and a base
B) water and a salt
C) water and carbon dioxide
D) a salt and carbon dioxide
E) a salt and hydrogen
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 10.0 mL of 0.121 M H2SO4 is neutralized by 17.1 mL of KOH solution.The molarity of the KOH solution is ________.
A) 0.207 M
B) 0.414 M
C) 0.0708 M
D) 0.428 M
E) 0.142 M
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The name of H2SO4 is hydrosulfuric acid.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The [ H3O+ ] of a solution with pH = 8.7 is ________.
A) 8.7 M
B) 5.3 M
C) 2 × ![]() M
M
D) 8.7 × ![]() M
M
E) 5 × ![]() M
M
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
25.0 mL of 0.212 M NaOH is neutralized by 13.6 mL of an HCl solution.The molarity of the HCl solution is ________.
A) 0.212 M
B) 0.115 M
C) 0.500 M
D) 0.390 M
E) 0.137 M
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) ![]()
![]()
B) ![]()
C) NaOH
D) ![]()
![]()
E) HCl
G) C) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The conjugate base of H20 IS H3O+.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a solution with [ H3O+ ] = 1 × ![What is the pH of a solution with [ H<sub>3</sub>O<sup>+</sup> ] = 1 × M? A) 1.0 × B) -9.0 C) 5.0 D) -5.0 E) 9.0](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_f6d9_91e5_d16976f0139a_TB6077_11.jpg) M?
M?
A) 1.0 × ![]()
B) -9.0
C) 5.0
D) -5.0
E) 9.0
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the [ ![What is the [ ] in a solution that has a [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_819e_91e5_f7d058637f0f_TB6077_11.jpg) ] in a solution that has a [
] in a solution that has a [ ![What is the [ ] in a solution that has a [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_a8af_91e5_41b8a5cf94dd_TB6077_11.jpg)
![What is the [ ] in a solution that has a [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_a8b0_91e5_e1d2d59d365e_TB6077_11.jpg) ] = 1 ×
] = 1 × ![What is the [ ] in a solution that has a [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_a8b1_91e5_6fe48da77371_TB6077_11.jpg) M?
M?
A) 1 × ![]() M
M
B) 1 × ![]() M
M
C) 1 × ![]() M
M
D) 1 × ![]() M
M
E) 1 × ![]() M
M
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Normal blood pH is about ________.
A) 6.8
B) 7.0
C) 7.2
D) 7.4
E) 7.6
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
When more reactant is added to a system iat equilibrium,the reverse reaction is favored.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
KOH is a strong base.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name given to an aqueous solution of HBr is ________.
A) hydrogen bromide
B) hydrobromic acid
C) bromic acid
D) bromous acid
E) hypobromous acid
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
HBr is a strong acid.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the [ ![What is the [ ] in a solution with [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_3375_91e5_5d7f4cf2f3b9_TB6077_11.jpg)
![What is the [ ] in a solution with [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_5a86_91e5_bbdf1e3c462a_TB6077_11.jpg) ] in a solution with [
] in a solution with [ ![What is the [ ] in a solution with [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_5a87_91e5_a1365084a03c_TB6077_11.jpg) ] = 1 ×
] = 1 × ![What is the [ ] in a solution with [ ] = 1 × M? A) 1 × M B) 1 × M C) 1 × M D) 1 × M E) 1 × M](https://d2lvgg3v3hfg70.cloudfront.net/TB6077/11ea71d0_de4f_5a88_91e5_bdf249c09fa1_TB6077_11.jpg) M?
M?
A) 1 × ![]() M
M
B) 1 × ![]() M
M
C) 1 × ![]() M
M
D) 1 × ![]() M
M
E) 1 × ![]() M
M
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of 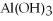 is ________.
is ________.
A) aluminum trihydroxide
B) monoaluminum trihydroxide
C) aluminum hydroxide
D) aluminum(III) hydroxide
E) aluminum oxygen hydride
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The conjugate acid of H2O is ________.
A) ![]()
![]()
B) ![]()
C) H2O
D) ![]()
E) H2O has no conjugate acid.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is correctly identified?
A) ![]() ,strong acid
,strong acid
B) NaOH,strong base
C) HCl,weak acid
D) ![]()
![]() ,strong acid
,strong acid
E) Ca ![]() ,weak base
,weak base
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 65
Related Exams