B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
N,N-diethyl-m-tolumide (DEET) is the active ingredient in many mosquito repellents. What is the hybridization state of carbon indicated by the arrow in the structure of DEET shown below 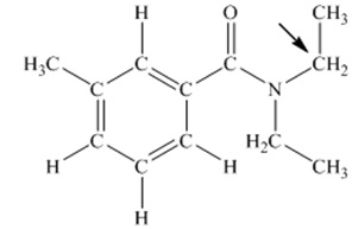
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Pi bonds are covalent bonds in which the electron density is concentrated above and below a plane containing the nuclei of the bonding atoms.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules is polar
A) PBr5
B) CCl4
C) BrF5
D) XeF2
E) XeF4
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules is nonpolar
A) NH3
B) OF2
C) CH3Cl
D) H2O
E) BeCl2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following would the bonding be weakened with the addition of an electron to form the negative molecular ion
A) B2
B) C2
C) N2
D) all of these
E) none of these
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the bond order of Cl2-
A) 0
B) 0.5
C) 1
D) 1.5
E) 2
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below 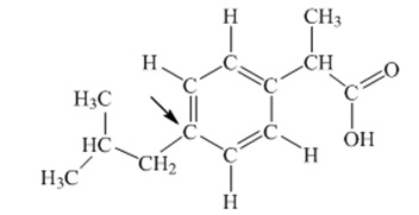
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules has tetrahedral geometry
A) XeF4
B) BF3
C) AsF5
D) CF4
E) NH3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An sp3 hybridized central carbon atom with no lone pairs of electrons has what type of bonding
A) 1 and 2 bonds
B) 1 and 3 bonds
C) 2 and 2 bonds
D) 3 and 2 bonds
E) 0 and 4 bonds
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 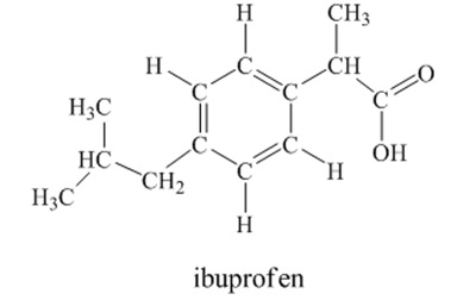 How many sigma bonds and pi bonds are contained in an ibuprofen molecule
33 sigma bonds and 4 pi bonds
How many sigma bonds and pi bonds are contained in an ibuprofen molecule
33 sigma bonds and 4 pi bonds
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The F - S - F bond angles in SF6 are
A) 90 and 180 .
B) 109.5 .
C) 120 .
D) 180 .
E) 90 and 120 .
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with 3 single bonds (and any number of lone pairs) could have which of the following molecular geometries I. Trigonal planar II. Trigonal pyramidal III. Seesaw IV. T-shaped
A) I and II
B) II and III
C) II and IV
D) I and III
E) I, II, and IV
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
A bonding molecular orbital is of lower energy (more stable) than the atomic orbitals from which it was formed.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The N - N - H bond angles in hydrazine N2H4 are 112 . What is the hybridization of the nitrogen orbitals predicted by valence bond theory
A) sp
B) sp2
C) sp3
D) sp4
E) None of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sp hybridized terminal oxygen atom (bonded to one other atom only) with 1 lone pair of electrons has what type of bonding
A) 1 and 2 bonds
B) 2 and 1 bonds
C) 2 and 2 bonds
D) 0 and 2 bonds
E) 0 and 3 bonds
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The BrF5 molecule has polar bonds and has a net dipole moment.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly lists species in order of increasing bond length
A) O2 < O2+ < O2-
B) O2- < O2 < O2+
C) O2+ < O2 < O2-
D) O2- < O2+ < O2
E) O2+ < O2- < O2
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
N,N-diethyl-m-tolumide (DEET) is the active ingredient in many mosquito repellents. 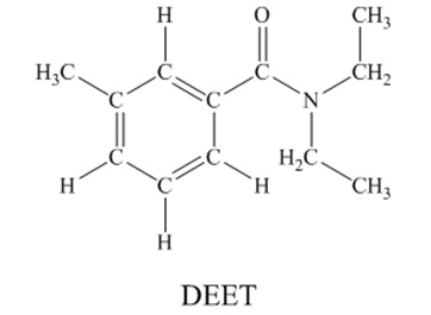 There are 30 sigma bonds and 4 pi bonds are contained in a DEET molecule.
There are 30 sigma bonds and 4 pi bonds are contained in a DEET molecule.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Ozone (O3) is an allotropic form of oxygen. According to VSEPR theory the shape of the ozone molecule is bent.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 146
Related Exams