A) O
B) S
C) Na
D) C
E) N
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of these species does the best Lewis structure have two or more equivalent resonance structures
A) HCO2-
B) SCN-
C) CNO-
D) N3-
E) CO2
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The bond in F2 is described as polar covalent.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds utilizes both ionic and covalent bonding
A) CO32-
B) Al2(SO4) 3
C) CO2
D) C6H12O6
E) MgCl2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The structure below depicts the correct Lewis structure for Dichloromethane, CH2Cl2 (an important solvent in synthetic chemistry). 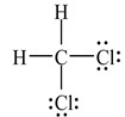
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the bond enthalpy data given to estimate the heat released when 50.0 g of propane gas, C3H8, burns in excess oxygen to yield carbon dioxide and water vapor at 25 C. BE(C-C) = 347 kJ/mol BE(C=O in CO2) = 799 kJ/mol BE(C-H) = 414 kJ/mol BE(O-H) = 460 kJ/mol BE(O=O) = 498.7 kJ/mol
A) 1360 kJ
B) 1540 kJ
C) 1970 kJ
D) 2240 kJ
E) 2370 kJ
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of lone electron pairs in the N2 molecule is ___.
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming the octet rule is obeyed, how many covalent bonds will an oxygen atom form to give a formal charge of zero
A) 0
B) 1
C) 2
D) 3
E) 4
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming the octet rule is obeyed, how many covalent bonds will a nitrogen atom form to give a formal charge of zero
A) 0
B) 1
C) 2
D) 3
E) 4
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the bond enthalpy data given to estimate the heat released when 25.0 g of acetylene gas, C2H2, burns in excess oxygen to yield carbon dioxide and water vapor at 25 C. BE(C-C) = 347 kJ/mol BE(C C) = 812 kJ/mol BE(C=O in CO2) = 799 kJ/mol BE(C-H) = 414 kJ/mol BE(O-H) = 460 kJ/mol BE(O=O) = 498.7 kJ/mol
A) 16.8 kJ
B) 364 kJ
C) 447 kJ
D) 1180 kJ
E) 1230 kJ
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the elements C, O, and H in order of increasing electronegativity.
A) C < O < H
B) H < C < O
C) C < H < O
D) O < C < H
E) H < O < C
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
A molecule with two resonance structures is shifting quickly back and forth from one structure to the other.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of lone electron pairs in the ClO4- ion is ___.
A) 3
B) 4
C) 6
D) 12
E) 24
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron dot formula for O2 shows
A) a single covalent bond.
B) a double covalent bond.
C) an ionic bond.
D) a total of 8 x 2 = 16 electron dots.
E) a total of 32 electron dots.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge on the central nitrogen atom in the most favorable Lewis structure for the fulminate ion, CNO-, based on minimizing formal charge overall
A) +2
B) +1
C) 0
D) -1
E) -2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many resonance phosphate Lewis structures can be drawn for the phosphate ion, PO43-, that obey the octet rule
A) One
B) Two
C) Three
D) Four
E) None of the above are correct
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ionic solids would have the largest lattice energy
A) KF
B) KI
C) LiF
D) LiI
E) NaF
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules has an atom with an incomplete octet
A) NF3
B) H2O
C) AsCl3
D) GeH4
E) BF3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Shown here is a Lewis structure for the chlorate ion, ClO3-, that obeys the octet rule, showing all non-zero formal charges. How many resonance structures for ClO3- are possible that obey the octet rule 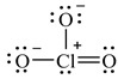
A) Four
B) Three
C) two
D) One
E) None of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The total number of valence electrons in the ion NH4+ is
A) 8.
B) 9.
C) 10.
D) 17.
E) 18.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 136
Related Exams