A) trigonal planar
B) trigonal pyramidal
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does the abbreviation VSEPR stand for?
A) Very Specific Electron and Proton Repair
B) Variable Selective of Electron and Protons
C) Valence Shell for Every Proton
D) Very Selective Electron Pair theory
E) Valence-Shell Electron-Pair Repulsion
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest intermolecular interactions between hydrogen fluoride (HF) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) ionic bonds.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model, a molecule with the general formula AB2 with two lone pairs on the central atom will have a _____ molecular geometry.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) seesaw
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predicted molecular geometry of the CH4 molecule according to the VSEPR model?
A) tetrahedral
B) trigonal pyramidal
C) trigonal planar
D) square planar
E) seesaw
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest intermolecular interactions between ethyl alcohol (CH3CH2OH) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) carbon-oxygen bonds.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules is polar?
A) CH4
B) CHBr3
C) F2
D) CBr4
E) CO2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of BeH2 as predicted by the VSEPR model?
A) tetrahedral
B) bent
C) trigonal planar
D) linear
E) trigonal pyramidal
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of HOF as predicted by the VSEPR model?
A) trigonal pyramidal
B) bent
C) tetrahedral
D) linear
E) trigonal planar
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is wrong with this Lewis structure? 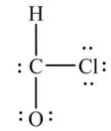
A) There are too many electrons.
B) There are too few electrons.
C) The O atom does not have an octet.
D) The C atom does not have an octet.
E) There is nothing wrong with this Lewis structure.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the number of lone electron pairs on the central atom of a molecule having a trigonal pyramidal molecular geometry, such as NH3?
A) 1
B) 2
C) 3
D) 0
E) 4
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model, the predicted molecular geometry of the SO3 molecule is
A) pyramidal.
B) tetrahedral.
C) trigonal planar.
D) seesaw.
E) square planar.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for nitrogen trifluoride, NF3.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model, predict the molecular geometry around the central atom in PO43−.
A) trigonal planar
B) trigonal pyramidal
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure for CS2 is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the number of lone electron pairs on the central atom of a molecule having a linear molecular geometry, such as ClF2−?
A) 1
B) 2
C) 3
D) 0
E) 4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following is the best Lewis structure a resonance structure?
A) SO3
B) BF3
C) I3−
D) SCO (C = central atom)
E) SO32−
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following pure substances are the intermolecular interactions entirely due to dispersion forces?
A) C2H6
B) CH3OCH3
C) NO2
D) H2S
E) Ca(NO3) 2
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which substance will exhibit hydrogen bonding between molecules?
A) (CH3) 3N
B) CH3-O-CH3
C) CH3CH2-OH
D) CH3CH2-F
E) HI
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predicted O-C-O bond angle in CO2?
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 71
Related Exams