A) 1.
B) 2.
C) 3.
D) 5.
E) 9.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the geometry around the central atom in SO42-.
A) trigonal planar
B) trigonal pyramidal
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Using periodic trends, arrange the following molecules in order of increasing dipole moment: NH3, PH3, AsH3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the molecular geometry and polarity of the SO2 molecule.
A) linear, polar
B) linear, nonpolar
C) bent, polar
D) bent, nonpolar
E) None of the above.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules has a non-zero dipole moment?
A) BeCl2
B) Br2
C) BF3
D) IBr
E) CO2
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The hybridization of B in the BF3 molecule is sp3.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the species N2-, N2, and N2+. Which of these species will be paramagnetic?
A) N2 and N2-
B) N2+ and N2
C) N2+ and N2-
D) only N2-
E) none are paramagnetic
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
N,N-diethyl-m-tolumide (DEET) is the active ingredient in many mosquito repellents. What is the hybridization state of the nitrogen atom in the structure of DEET shown below? 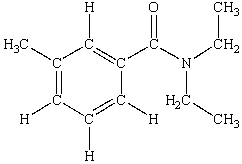
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, which one of the following molecules has tetrahedral geometry?
A) NH3
B) CCl4
C) CO2
D) SF4
E) PCl5
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR theory, the actual F -As -F bond angles in the AsF4- ion are predicted to be
A) 109.5°.
B) 90° and 120°.
C) 180°.
D) < 109.5°.
E) < 90° and < 120°.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
N,N-diethyl-m-tolumide (DEET) is the active ingredient in many mosquito repellents. What is the hybridization state of carbon indicated by the arrow in the structure of DEET shown below? 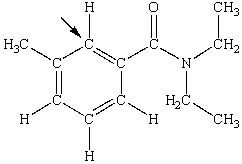
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the geometry and polarity of the CS2 molecule.
A) linear, polar
B) linear, nonpolar
C) tetrahedral, nonpolar
D) bent, nonpolar
E) bent, polar
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of As in the AsF4- ion?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate the type of hybrid orbitals used by the central atom in PCl3.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The F - S - F bond angles in SF6 are
A) 90° and 180°.
B) 109.5°.
C) 120°.
D) 180°.
E) 90° and 120°.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the central nitrogen atom in the molecule N2O is
A) sp.
B) sp2.
C) sp3.
D) sp3d.
E) sp3d2.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Which of the following molecules should be polar? a. CH3OH b. H2O c. CH3OCH3
Correct Answer

verified
All of the...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The number of pi bonds in the oxalate ion (C2O42-) is
A) 1.
B) 2.
C) 3.
D) 4.
E) 5.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the As atom in the AsF5 molecule?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Pi bonds are covalent bonds in which the electron density is concentrated above and below a plane containing the nuclei of the bonding atoms.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 120
Related Exams