A) hydriodic acid.
B) hydroiodous acid.
C) iodic acid.
D) iodous acid.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction 2 H2O2(l) → 2 H2O(l) + O2(g) oxygen is
A) both oxidized and reduced.
B) neither oxidized nor reduced.
C) only oxidized.
D) only reduced.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The substance is undergoing oxidation in the reaction below is ________. 2 S2O32-(aq)+ I3-(aq)→ S4O62-(aq)+ 3 I-(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of the sulfur atom in S8?
A) -2
B) 0
C) +6
D) +8
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When K2SO4(aq) and Pb(NO3) 2(aq) are mixed,a white colored precipitate forms which is
A) KNO3.
B) K2SO3.
C) Pb.
D) PbSO4.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which elements will not react with liquid water but will react with aqueous H+ ions?
A) Ag,Cu,Hg,Pt
B) Al,Cr,Mn,Zn
C) Ba,Ca,Li,Na
D) Ag,Au,Ca,K
F) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
The hydrogen ion,H+,is also referred to as a ________,and a hydrated hydrogen ion,H3O+,is called a ________ ion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What reagent could be used to separate Br- from NO3- when added to an aqueous solution containing both?
A) AgNO3(aq)
B) Ba(OH) 2(aq)
C) CuSO4(aq)
D) NaI(aq)
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assume that an aqueous solution of a cation,represented by shaded spheres,is allowed to mix with a solution of an anion,represented by unshaded spheres.Three possible outcomes are represented by boxes (a) -(c) . 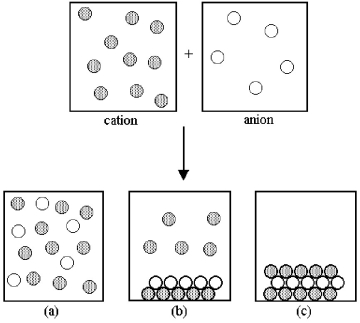 -Which outcome corresponds to the combination of copper(II) and sulfide ions shown in the following equation?
Cu2+(aq) + S2-(aq) → ?
-Which outcome corresponds to the combination of copper(II) and sulfide ions shown in the following equation?
Cu2+(aq) + S2-(aq) → ?
A) box (a)
B) box (b)
C) box (c)
D) None of these
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phthalic acid is a diprotic acid having the formula HO2CC6H4CO2H that can be converted to a salt by reaction with base.Which of the following is expected to be most soluble in water?
A) HO2CC6H4CO2H
B) HO2CC6H4CO2Na
C) HO2CC6H4CO2K
D) NaO2CC6H4CO2K
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assume that an aqueous solution of hydroxide ion,OH-,represented by unshaded spheres,is allowed to mix with a solution of an acid,HnA,represented by gray spheres.Three possible outcomes are represented by boxes (a) -(c) ,where the black spheres represent An-,the anion of the acid.For clarity H2O molecules are not shown. 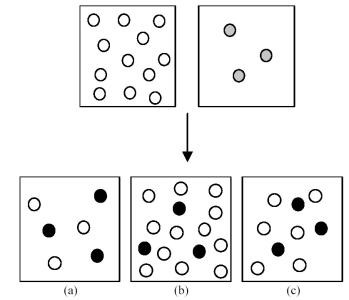 -Which outcome corresponds to the reaction:
HCN + OH- → H2O + CN-?
-Which outcome corresponds to the reaction:
HCN + OH- → H2O + CN-?
A) box (a)
B) box (b)
C) box (c)
D) None of these
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the number of water molecules necessary to balance the reduction half reaction of ____ MnO4-(aq) → ____ MnO2(s) that occurs in a basic solution.
A) 2
B) 3
C) 4
D) 5
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Because it forms some H+ and OCl- ions when dissolved in water,the molecule HOCl is classified as a(n)________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What reagent could not be used to separate Br- from CO32- when added to an aqueous solution containing both?
A) AgNO3 (aq)
B) Ca(NO3) 2 (aq)
C) Cu(NO3) 2 (aq)
D) Fe(NO3) 2(aq)
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of compounds is soluble in water?
A) AgBr and AgI
B) CdS and (NH4) 2S
C) KI and Ba(NO3) 2
D) NaNO3 and CuCO3
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the balanced chemical equation 5 H2C2O4(aq) + 2 MnO4-(aq) + 6 H+(aq) → 10 CO2(g) + 2 Mn2+(aq) + 8 H2O(l) 0) 3500 grams of oxalic acid,H2C2O4 will react with ________ mL of 0.100 M potassium permanganate,KMnO4 solution.
A) 15.5 mL
B) 38.9 mL
C) 77.7 mL
D) 97.2 mL
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Metals that do not react with hydrochloric acid to produce hydrogen gas are found ________ H2 in the activity series.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mixing of which pair of reactants will result in a precipitation reaction?
A) CsI(aq) + NaOH(aq)
B) HCl(aq) + Ca(OH) 2(aq)
C) K2SO4(aq) + Hg2(NO3) 2(aq)
D) NaNO3(aq) + NH4Cl(aq)
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar concentration of sodium ions in a 0.350 M Na3PO4 solution?
A) 0) 117 M
B) 0) 350 M
C) 1) 05 M
D) 1) 40 M
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which compound is the oxidation state of hydrogen not +1?
A) H2O
B) H2O2
C) NaH
D) Na2HSO4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 174
Related Exams