A) only NaCl(aq)
B) only HNO3(aq)
C) HCl(aq) or NaCl(aq)
D) only HCl(aq)
E) HCl(aq) or HNO3(aq)
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of calcium carbonate in water at 25°C is 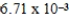 g/L.What is the Ksp of this sparingly soluble salt?
g/L.What is the Ksp of this sparingly soluble salt?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following salts has the lowest molar solubility in water?
A) Ni(OH) 2 (Ksp = 2.0 × 10-15)
B) Fe(OH) 2 (Ksp = 8 × 10-16)
C) PbI2 (Ksp = 6.5 × 10-9)
D) CaCO3 (Ksp = 3.8 × 10-9)
E) AgBr (Ksp = 5.0 × 10-13)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose sodium hydroxide is added to a 0.0042 M solution of zinc nitrate such that the pH of the solution is 13.04.What is the equilibrium concentration of Zn2+?
Zn2+(aq) + 4OH-(aq)  Zn(OH) 42-(aq) ; Kf = 2.8 × 1015
Zn(OH) 42-(aq) ; Kf = 2.8 × 1015
A) 1.6 × 10-17 M
B) 4.2 × 10-3 M
C) 2.7 × 10-2 M
D) 1.0 × 10-14 M
E) 1.4 × 10-17 M
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the solubility product expression for Pb(IO3) 2?
A) Ksp = [Pb2+][IO3-]2
B) Ksp = [Pb4+][2IO32-]2
C) Ksp = [Pb2+][2IO3-]
D) Ksp = [Pb4+][IO32-]2
E) Ksp = [Pb2+][2IO3-]2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the qualitative analysis scheme for metal ions,how are the Analytical Group III cations separated from the cations of Analytical Groups IV and V?
A) by addition of H2S in acidic solution,forming insoluble metal sulfides
B) by addition of (NH4) 2CO3 or (NH4) 3PO4,forming insoluble metal carbonates or phosphates
C) by addition of H2SO4,forming insoluble metal sulfates
D) by addition of HCl,forming insoluble metal chlorides
E) by addition of H2S in basic solution,forming insoluble metal sulfides or hydroxides
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions should be added to a solution containing both copper(II) ions and silver(I) ions in order to precipitate only one of the ions?
A) HCl(aq)
B) H2S(aq)
C) HNO3(aq)
D) H2S(aq) + HCl(aq)
E) H2S(aq) + HNO3(aq)
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents a step in the separation of which analytical group of cations? Cu2+(aq) + S2-(aq) → CuS(s)
A) Analytical Group I
B) Analytical Group III
C) Analytical Group V
D) Analytical Group IV
E) Analytical Group II
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar equilibrium concentration of uncomplexed Fe2+(aq) in a solution composed of 1.4 mol Fe(CN) 64− dissolved in 1.00 L of 0.33 M NaCN.Kf for Fe(CN) 64− is 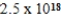 .
.
A) ![]() M
M
B) ![]() M
M
C) ![]() M
M
D) ![]() M
M
E) ![]() M
M
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following salts in order of increasing molar solubility.
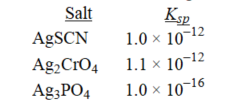
A) AgSCN < Ag2CrO4 < Ag3PO4
B) AgSCN < Ag3PO4 < Ag2CrO4
C) Ag3PO4 < Ag2CrO4 < AgSCN
D) Ag3PO4 < AgSCN < Ag2CrO4
E) Ag2CrO4 < AgSCN < Ag3PO4
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Pure water is saturated with slightly soluble calcium fluoride,CaF2.Which of the following is true concerning the equilibrium concentration of Ca2+?
A) ![]()
B) [Ca2+] = [F-]
C) ![]()
D) ![]()
E) [Ca2+] = Ksp
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cation C and anion A form an ionic compound for which Ksp = s2,where s is the molar solubility of the ionic compound.Which of Figures I-III represent(s) possible results of the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A? 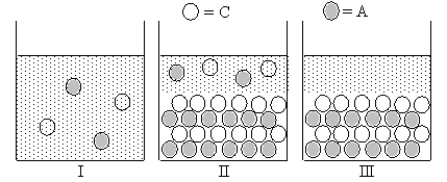
A) only I
B) only III
C) both I and III
D) both I and II
E) only II
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ksp for PbF2 is 4.0 ×10-8.If a 0.032 M NaF solution is saturated with PbF2,what is [Pb2+] in solution?
A) 4.1 × 10-11 M
B) 1.3 × 10-9 M
C) 1.3 × 10-6 M
D) 1.2 × 10-3 M
E) 3.9 × 10-5 M
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A(n) _____ is a Lewis base that bonds to a metal ion to form a complex ion.
A) ligand
B) zwitterion
C) substrate
D) alkane
E) arene
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sparingly soluble salt will exhibit the highest solubility at low pH's?
A) PbS (Ksp = 2.5 × 10-27)
B) MnS (Ksp = 2.5 × 10-10)
C) HgS (Ksp = 1.6 × 10-52)
D) NiS (Ksp = 3 × 10-9)
E) ZnS (Ksp = 1.1 × 10-21)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar solubility of MgF2 in a 0.40 M NaF solution? For MgF2,Ksp = 8.4 × 10-8.
A) 1.0 × 10-7 M
B) 1.4 × 10-4 M
C) 2.1 × 10-7 M
D) 7.1 × 10-4 M
E) 5.3 × 10-7 M
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following salts has the highest molar solubility in water?
A) CaCO3 (Ksp = 3.8 × 10-9)
B) Ni(OH) 2 (Ksp = 2.0 × 10-15)
C) Fe(OH) 2 (Ksp = 8 × 10-16)
D) AgBr (Ksp = 5.0 × 10-13)
E) PbI2 (Ksp = 6.5 × 10-9)
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of lead(II) sulfate is 4.0 × 10-2 g/L.What is the solubility product constant for lead(II) sulfate?
A) 1.7 × 10-8
B) 1.3 × 10-4
C) 1.6 × 10-3
D) 4.6× 10-15
E) 8.9 × 10-12
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar solubility of MgF2 in a 0.36 M Mg(NO3) 2 solution? For MgF2,Ksp = 8.4 × 10-8.
A) 8.0 × 10-8 M
B) 2.4 × 10-4 M
C) 2.0 × 10-8 M
D) 4.8 × 10-4 M
E) 3.2 × 10-3 M
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the solubility (in g/L) of barium chromate at 25°C? The solubility product constant for barium chromate is 1.2 × 10-10 at 25°C.
A) 0.41 g/L
B) 3.0 × 10-8 g/L
C) 1.5 × 10-8 g/L
D) 0.078 g/L
E) 0.0027 g/L
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 115
Related Exams