A) The electron is captured by the nucleus,which becomes unstable.
B) The nuclides produced are more stable than the uranium nuclide.
C) The nuclides produced are individually heavier than the uranium nuclide.
D) The products include neutrons.
E) Two of these statements are true.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 210Pb has a half-life of 22 years.What percentage of a pure 210Pb sample prepared in April 1937 remains in April 1994?
A) 17%
B) 26%
C) 31%
D) 21%
E) 38%
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Relative to the sum of the masses of its constituent nucleons (neutrons + protons) ,the mass of a nucleus is
A) always greater.
B) sometimes the same and sometimes smaller.
C) always smaller.
D) always the same.
E) sometimes greater and sometimes smaller.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iodine-131,which is used to treat thyroid cancer,has a half-life of 8.04 days.How much time is required for 68% of the isotope to decay?
A) 13 days
B) 7 days
C) 43 days
D) 53 days
E) 4 days
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Metastable isotopes,such as Technetium-99m and those produced from neutron activation analysis decay by what process?
A) gamma emission
B) alpha emission
C) beta emission
D) positron emission
E) all of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about 248Bk is incorrect?
A) If 248Bk were to undergo spontaneous fission,the products would be 247Bk and a neutron.
B) If 248Bk were to undergo beta decay,the products would be 248Cf and a beta particle.
C) If 248Bk were to undergo alpha decay,the products would be 244Am and an alpha particle.
D) If 248Bk were to undergo electron capture,the only product would be 248Cm.
E) If a metastable form of 248Bk were to undergo gamma decay,the products would be 248Bk and a gamma ray.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following nuclides will produce 233Pa upon undergoing alpha decay?
A) (234Pa)
B) (233U)
C) (229Ra)
D) (237Np)
E) (233Th)
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 235U collides with one neutron,fission occurs.What is one possible set of products?
A) four neutrons,90Sr,and 140Xe
B) four neutrons,90Sr,and 139Xe
C) four neutrons,90Sr,and 139Ce
D) four neutrons,90Sr,and 141Xe
E) four neutrons,90Sr,and 142Xe
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The gamma ray emission from the decay of cobalt-60 is used in cancer therapies.Cobalt-60 decays by the emission of two gamma rays followed by beta emission.What is the final product of this decay process?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 103Pd undergoes electron capture,what is the product nuclide?
A) (103Ag)
B) (101Rh)
C) (107Ag)
D) (103Rh)
E) (104Cd)
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What assumption must be true for radiocarbon (14C) dating to be useful?
A) (14C) has the same mass as 12C.
B) A constant concentration of 14C is maintained in living plants and animals through equilibration with atmospheric levels of 14C.
C) The sample cannot have been chemically altered prior to the analysis.
D) (14C) always decays at the same rate.
E) (14C) is nonradioactive.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following particles cannot be accelerated to high speeds in a particle accelerator or cyclotron?
A) neutrons
B) electrons
C) protons
D) alpha particles
E) hydrogen nuclei
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the decay constant for a particular radioactive element that has a half-life of 5.66 years?
A) 0.161/year
B) 0.113/year
C) 7.18 × 10-3/h
D) 25.8/s
E) 0.122/year
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following nuclides will produce 192Pt upon undergoing beta decay?
A) (192Ir)
B) (193Pt)
C) (192Au)
D) (196Hg)
E) (188Os)
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a certain type of nucleus that has a rate constant of 2.43 × 10-2 min-1.Calculate the time required for the sample to decay to one-fourth of its initial value.
A) 28.5 min
B) 35.6 min
C) 0.0486 min
D) 57.0 min
E) 2.43 min
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most likely mode of decay for the radioactive nuclide 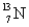 ?
?
A) positron emission
B) gamma radiation
C) beta emission
D) alpha emission
E) neutron emission
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of wood from an Egyptian mummy case gives a 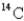 count of 8.5 cpm/gC (counts per minute per gram of carbon) .How old is the wood? (The initial decay rate of
count of 8.5 cpm/gC (counts per minute per gram of carbon) .How old is the wood? (The initial decay rate of 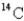 Is 15.3 cpm/g C,and its half-life is 5730 years.)
Is 15.3 cpm/g C,and its half-life is 5730 years.)
A) ![]() years
years
B) ![]() years
years
C) ![]() years
years
D) ![]() years
years
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a neutron in the nucleus is converted to a proton,which of the following is emitted?
A) positron
B) beta particle
C) alpha particle
D) deuteron
E) neutron
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these is not a transuranium element?
A) plutonium
B) neptunium
C) curium
D) thorium
E) americium
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide 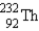 is radioactive.When one of these atoms decays,a series of α and β- particle emissions occurs,taking the atom through many transformations to end up as an atom of
is radioactive.When one of these atoms decays,a series of α and β- particle emissions occurs,taking the atom through many transformations to end up as an atom of 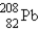 ) How many α particles are emitted in converting
) How many α particles are emitted in converting 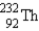 Into
Into 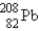 ?
?
A) 6
B) 2
C) 214
D) 8
E) 4
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 90
Related Exams