A) 0 lone pairs, linear
B) 1 lone pair, bent
C) 2 lone pairs, bent
D) 3 lone pairs, bent
E) 3 lone pairs, linear
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to predict the geometry of the PCl3 molecule.
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
According to VSEPR theory, which of the following triatomic ions should be linear: N3-, I3-, NO2-, ClO2-, SCN-.
Correct Answer

verified
N3-, I3-, and ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following molecules has tetrahedral geometry?
A) XeF4
B) BF3
C) AsF5
D) CF4
E) NH3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
A bonding molecular orbital is of lower energy (more stable)than the atomic orbitals from which it was formed.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules is nonpolar?
A) NH3
B) OF2
C) CH3Cl
D) H2O
E) BeCl2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the geometry of the ion ClO3-.
A) 0 lone pairs, trigonal
B) 1 lone pair, bent
C) 1 lone pair, trigonal pyramidal
D) 2 lone pairs, T-shaped
E) 2 lone pairs, trigonal
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond angle in ICl2- is expected to be
A) a little less than 109.5°.
B) 109.5°.
C) a little more than 109.5°.
D) 120°.
E) 180°.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
According to the VSEPR theory, all of the electron pair-electron pair repulsions about the central atom in PCl3 are of equal magnitude.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly lists species in order of increasing bond order?
A) C2 < Li2 < Be2 < N2
B) Be2 < Li2 < C2 < N2
C) N2 < Be2 < Li2 < C2
D) N2 < C2 < Li2 < Be2
E) Be2 < C2 < N2 < Li2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever.What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 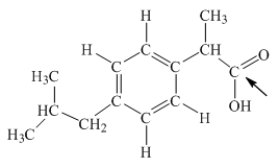
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the molecular geometry of CBr4.
A) 0 lone pairs, square planar
B) 0 lone pairs, tetahedral
C) 1 lone pair, square pyramidal
D) 1 lone pair, trigonal bipyramidal
E) 2 lone pairs, square planar
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules has a non-zero dipole moment?
A) BeCl2
B) Br2
C) BF3
D) IBr
E) CO2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR theory, the actual F -As -F bond angles in the AsF4- ion are predicted to be
A) 109.5°
B) 90° and 120°
C) 180°
D) < 109.5°
E) < 90° and < 120°
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
More energy is required to break a bond with an order of 3/2 than is required to break a bond of order 2.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly lists species in order of increasing bond length?
A) O2 < O2+ < O2-
B) O2- < O2 < O2+
C) O2+ < O2 < O2-
D) O2- < O2+ < O2
E) O2+ < O2- < O2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the molecular geometry of XeF4.
A) 0 lone pairs, tetrahedral
B) 1 lone pair, distorted tetrahedron (seesaw)
C) 1 lone pair, square pyramidal
D) 1 lone pair, tetrahedral
E) 2 lone pairs, square planar
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever.What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 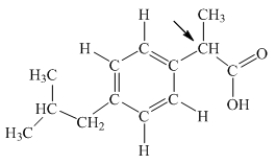
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Complete the following table. 
Correct Answer

verified
Correct Answer
verified
Short Answer
Indicate the number of -bonds in C2H6.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 122
Related Exams