A) 6.02 × 1023 molecules of Cl2
B) 35.45 g of Cl2
C) 0.500 mol of Cl2
D) All of these have the same mass.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of CuO can be produced from 1.80 mol of Cu2O in the following reaction? 2 Cu2O(s) + O2(g) → 4 CuO(s)
A) 0.900 mol
B) 1.80 mol
C) 3.60 mol
D) 7.20 mol
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
3.0 moles of nitrogen is reacted with 11.0 moles of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is false? N2(g) + 3 H2(g) → 2 NH3(g)
A) 2.0 moles of hydrogen are left over.
B) Hydrogen is the excess reactant.
C) Nitrogen is the limiting reactant.
D) 12.0 moles of ammonia are produced.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 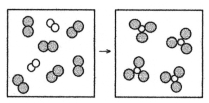
A) A + B → AB
B) A + 3B → 2AB
C) A2 + B2 → AB3
D) A2 + 3B2 → 2AB3
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of sulfur hexafluoride,SF6,has the same number of fluorine atoms as 50.0 g of oxygen difluoride,OF2?
A) 202.7 g
B) 8.33 g
C) 43.0 g
D) 135.1 g
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrazine,N2H4,once used as a rocket propellant,reacts with oxygen in the following equation. N2H4(g) + O2(g) → N2(g) + 2 H2O(g) The reaction,at 50% yield,produces 4.0 moles of N2.What was the mass of hydrazine used? Assume that O2 is in excess.
A) 32.0 g
B) 260 g
C) 64 g
D) 0.063 g
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When iron(III) oxide reacts with hydrochloric acid,iron(III) chloride and water are formed.How many grams of iron(III) chloride are formed from 10.0 g of iron(III) oxide and 10.0 g of hydrochloric acid?
A) 11.1 g
B) 14.8 g
C) 20.3 g
D) 35.1 g
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Aluminum metal reacts with aqueous copper(II) sulfate to form aqueous aluminum sulfate and copper metal.What is the stoichiometric coefficient for aluminum when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients?
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the greatest mass?
A) 6.0 × 1023 atoms of O
B) 3.0 × 1023 molecules of O2
C) 2.0 × 1023 molecules of O3
D) All have the same mass.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of a single astatine molecule,At2?
A) 3.49 × 10-22 g
B) 6.97 × 10-22 g
C) 210 g
D) 420 g
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the percent yield for the following reaction is 65.0%,how many grams of KClO3 are needed to produce 42.0 g of O2? 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
A) 69.7 g
B) 107 g
C) 165 g
D) 371 g
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the empirical formula for perfluoropropane if the compound contains 81% fluorine and 19% carbon by mass?
A) CF3
B) C2F4
C) C3F8
D) C6F10
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 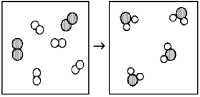
A) A + B → AB
B) 4A + 2B → 4AB
C) A2 + B2 → A2B
D) 2A2 + B2 → 2A2B
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following diagrams represent the reaction of A2 (shaded spheres) with B2 (unshaded spheres) .Identify the limiting reactant and write a balanced equation for the reaction. 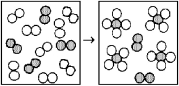
A) A2 is the limiting reactant;A + 4 B → AB4.
B) A2 is the limiting reactant;A2 + 4 B2 → 2 AB4.
C) B2 is the limiting reactant;A + 4 B → AB4.
D) B2 is the limiting reactant;A2 + 4 B2 → 2 AB4.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tablets of ascorbic acid,or Vitamin C,C6H8O6,are taken as a dietary supplement.If a typical tablet contains 500 mg,how many molecules of Vitamin C are in a tablet?
A) 500 molecules
B) 1.71 × 1024 molecules
C) 3.0 × 1024 molecules
D) 1.71 × 1021 molecules
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of pentane if 4.18 × 1016 molecules of pentane weigh 5.00 μg?
A) 72.0 g/mol
B) 139 g/mol
C) 288 g/mol
D) 347 g/mol
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
To the nearest whole number,the molar mass of Cu(NO3)2 is ________ g/mol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which substance is the limiting reactant when 16.0 g of sulfur reacts with 10.0 g of oxygen and 12.0 g of sodium hydroxide according to the following chemical equation? 2 S(s) + 3 O2(g) + 4 NaOH(aq) → 2 Na2SO4(aq) + 2 H2O(l)
A) S(s)
B) O2(g)
C) NaOH (aq)
D) Na2SO4(aq)
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 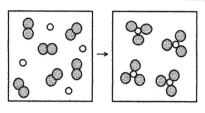
A) A + B → AB
B) 2A + 3B → 2AB
C) A + B2 → AB3
D) 2A + 3B2 → 2AB3
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of sulfur hexafluoride,SF6,has the same number of fluorine atoms as 25.0 g of oxygen difluoride,OF2?
A) 0.901 g
B) 8.33 g
C) 22.5 g
D) 203 g
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 159
Related Exams