A) a
B) b
C) c
D) a and b
E) all of the above
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the above molecules would be acidic?
A) a
B) b
C) c
D) d
E) a or d
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrocarbons release a lot of energy when ignited. Where does this energy ultimately come from?
A) the breaking of chemical bonds
B) the formation of chemical bonds
C) photosynthesis
D) nuclear fusion in the sun
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An amine can often form R-NH3+ (where R- can stand for anything) by reacting with water. What is happening?
A) The amine is accepting a proton from the water molecule, forming OH-.
B) Amines are basic.
C) Amines are acidic.
D) Amines can form H3O+ in water by donating a proton from the polar portion of the molecule.
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the above sections of this molecule would be considered an unsaturated portion of the molecule?
A) a
B) b
C) c
D) a and b
E) all of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the above molecules is a ketone?
A) a
B) b
C) c
D) d
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Formaldehyde is a toxic preservative with the following chemical formula: H2C 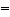 O Which of the following compounds would best serve as a starting point for its production?
O Which of the following compounds would best serve as a starting point for its production?
A) H3COH
B) H2C ![]() CH2
CH2
C) H2O
D) CH3CH2OH
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What determines the chemical and physical properties of hydrocarbons?
A) the way the atoms are connected together
B) the number of carbon and hydrogens
C) the elements it is composed of
D) the number of oxygen
E) both A and B
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An amino acid is an organic molecule that contains both an amine group and a carboxyl group. At an acidic pH, which structure is most likely: 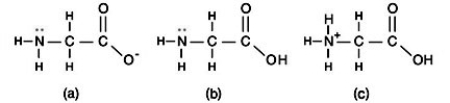
A) Structure (a)
B) Structure (b)
C) Structure (c)
D) All three structures are equally possible.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the percent volume of water in 80-proof vodka?
A) 80 percent
B) 60 percent
C) 40 percent
D) 20 percent
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an alcoholic beverage is 20 percent alcohol by volume, what is its proof?
A) 40 proof
B) 20 proof
C) 10 proof
D) Proof is a measure of flammability.
E) Proof is a measure of the age of a fermented beverage.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many structural isomers are shown here? 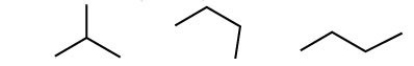
A) one
B) two
C) three
D) four
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an aromatic molecule? 
A) a
B) b
C) c
D) d
E) all of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Correctly identify the following functional groups in this organic molecule-amide, ester, ketone, ether, alcohol, aldehyde, amine. 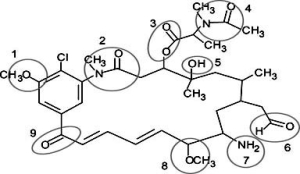
A) 1 = ether, 3 = ester, 6 = aldehyde, 9 = alcohol
B) 2 = amide, 4 = ester, 7 = amine, 8 = ether
C) 1 = ester, 5 = alcohol, 8 = ether, 9 = ketone
D) 2 = amide, 6 = aldehyde, 7 = amine, 8 = ether
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One solution to the problem of our overflowing landfills is to burn plastic objects instead of burying them. What would be some of the advantages and disadvantages of this practice?
A) disadvantage: toxic air pollutants; advantage: reduced landfill volume
B) disadvantage: loss of vital petroleum-based resource; advantage: generation of electricity
C) disadvantage: discourages recycling; advantage: provides new jobs
D) all of the above
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you saw the label "phenylephrine HCl" on a decongestant, would you worry that consuming it would expose you to the strong acid hydrochloric acid, HCl? 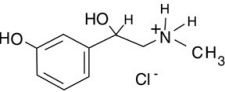
A) No, because it is balanced by the phenylephrine.
B) No, because the drug is in the solid phase.
C) No, because this is a salt made using hydrogen chloride, but it is in no way hydrogen chloride.
D) Yes, because of the hydrochloric acid it contains.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules would probably be isolated closest to the top of a fractionating tower at a refinery?
A) C4H10
B) C8H18
C) C10H22
D) C20H42
E) C40H82
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the chemical formula for the following structure? 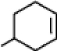
A) C6H10
B) C6H9
C) C7H12
D) C7H11
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 98 of 98
Related Exams