A) It has 4n electrons.
B) It is not cyclic.
C) It has 4n+2 electrons.
D) The electron system is not continuous.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 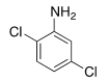
A) 3,6-Dichloroaniline
B) 2,5-Dichloroaniline
C) 2,5-Dichloroaminobenzene
D) 2,5-Dichloroanisole
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has the most acidic hydrogen atoms? 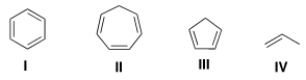
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following heterocycles is not aromatic? 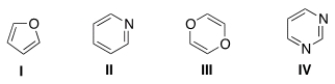
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is not aromatic? 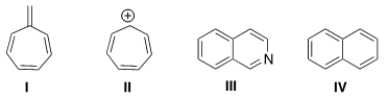
A) I
B) II
C) III
D) IV
F) B) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following statements about the structure of benzene is not true?
A) Benzene is planar.
B) Benzene has three short double bonds alternating with three longer single bonds.
C) The electrons in the pi bonds are delocalized around the ring.
D) Benzene has six pi electrons.
F) All of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following ions is aromatic? 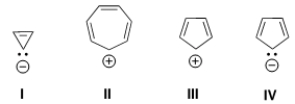
A) Only I and II
B) Only II and III
C) Only III and IV
D) Only II and IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a criterion for antiaromaticity?
A) The molecule must be cyclic.
B) The molecule must have (4n + 2) pi electrons.
C) The molecule must be planar.
D) The molecule must be completely conjugated.
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing acidity, starting with the most acidic. 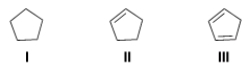
A) I > II > III
B) III > II > I
C) III > I > II
D) I > III > II
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the molecular orbital (MO) theory is true?
A) When two p orbitals of similar phase overlap side-by-side, a * antibonding molecular orbital is formed.
B) When two p orbitals of opposite phase overlap side-by-side, a bonding molecular orbital is formed.
C) A bonding molecular orbital is higher in energy than the two atomic p orbitals from which it is formed.
D) A * antibonding molecular orbital is higher in energy than the two atomic p orbitals from which it is formed.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct assignment of the names of the following aromatic heterocycles? 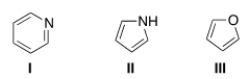
A) I = pyridine; II = pyrimidine; III = furan.
B) I = pyrimidine; II =pyrrole; III = furan.
C) I = pyridine; II = pyrrole; III = furan.
D) I = pyrimidine; II = pyrrole; III = tetrahydrofuran.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the following compound not aromatic? 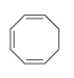
A) It has 4n pi electrons.
B) It is not cyclic.
C) It has 4n+2 pi electrons.
D) The electron system is not continuous.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct structure for anisole? 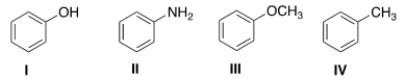
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is not aromatic? 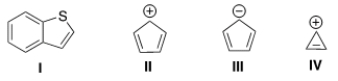
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the following compound? 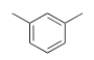
A) 2,6-dimethylbenzene
B) 5-methyltoluene
C) anisole
D) 1,3-dimethylbenzene
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What orbitals are used to form the indicated bond? 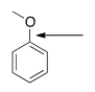
A) sp3
B) sp2
C) p
D) sp3 and sp2
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct assignment of the names of the following substituted benzenes? 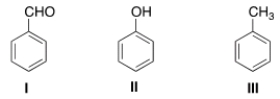
A) I = Phenol; II = anisole; III = toluene.
B) I = Benzaldehyde; II = phenol; III = anisole.
C) I = Benzaldehyde; II = phenol; III = toluene.
D) I = Phenol; II = anisole; III = styrene.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 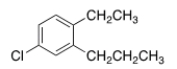
A) 1-Chloro-4-ethyl-3-propylbenzene
B) 1-Chloro-4-ethyl-5-propylbenzene
C) 4-Chloro-1-ethyl-2-propylbenzene
D) 4-Chloro-2-propyltoluene
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not one of the Hückel's criteria for aromaticity?
A) The molecule must be cyclic.
B) The molecule must be planar.
C) The molecule must be completely conjugated.
D) The molecule must have 4n pi electrons.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct assignment of the names of the following fused aromatic compounds? ![What is the correct assignment of the names of the following fused aromatic compounds? A) I = naphthalene; II = anthracene; III = phenanthrene. B) I = naphthalene; II = phenanthrene; III = anthracene. C) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene. D) I = anthracene; II = naphthalene; III = phenanthrene.](https://d2lvgg3v3hfg70.cloudfront.net/TB5871/11ea9088_70ba_4954_aec7_af90beedcffb_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) I = naphthalene; II = anthracene; III = phenanthrene.
B) I = naphthalene; II = phenanthrene; III = anthracene.
C) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene.
D) I = anthracene; II = naphthalene; III = phenanthrene.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 40
Related Exams