A) K
B) Te
C) Br
D) I
E) Rb
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for XeF4.
A) tetrahedral
B) bent
C) pyramidal
D) linear
E) none of these
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for PO33-.
A) tetrahedral
B) octahedral
C) square planar
D) pyramidal
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following Lewis structure: 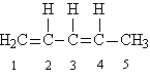 -How many unshared pairs of electrons are present in this molecule?
-How many unshared pairs of electrons are present in this molecule?
A) 1
B) 4
C) 0
D) 3
E) 2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As a general pattern, electronegativity is inversely related to
A) atomic size
B) polarity of the atom.
C) the number of neutrons in the nucleus.
D) ionization energy.
E) two of these.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound does not contain both polar covalent and ionic bonds?
A) Mg(CN) 2
B) NH4ClO2
C) C2H5OH
D) NaOH
E) RbC2H3O2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
How many lone pairs of electrons are around the central atom?
Correct Answer

verified
one lone p...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following series is isoelectronic?
A) B, C, N, O
B) Sn, As, S, F
C) Na, K, Rb, Cs
D) S2-, Cl-, K+, Ca2+
E) F-, Cl-, K+, Rb+
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Refer to the SeF4 molecule. -What are the angles of the Se-F bonds?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Estimate the bond energy of the N2 molecule. 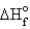 for NH3 = -46.0 kJ/mol N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
for NH3 = -46.0 kJ/mol N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
A) 1140 kJ/mol
B) 560 kJ/mol
C) 87 kJ/mol
D) 479 kJ/mol
E) 958 kJ/mol
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the species N2, CO, CN- and NO+ is false?
A) All are isoelectronic.
B) The bond in each species is polar.
C) All are linear.
D) Each contains a triple bond.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the molecule with the strongest bond.
A) HBr
B) HCl
C) HF
D) HI
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following compounds does the bond between the central atom and fluorine have the greatest ionic character?
A) SF2
B) SeF2
C) OF2
D) SbF3
E) AsF3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model, the electron pairs around NH3 and those around CH4 are arranged
A) differently, because in each case there are a different number of atoms around the central atom.
B) the same, because both nitrogen and carbon are in the second period.
C) differently, because in each case there are a different number of electron pairs around the central atom.
D) differently or the same, depending on the conditions leading to maximum repulsion.
E) the same, because in each case there are the same number of electron pairs around the central atom.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A quantum chemistry program indicates that the dipole moment of PH3 is zero. Which statement(s) best explains this result?
A) PH3 is a trigonal planar structure and the bond dipoles cancel.
B) PH3 violates the octet rule and does not exist.
C) The electronegativities of P and H are very close in value; therefore, no bond dipoles exist.
D) A and C only
E) A, B, and C
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is nonpolar?
A) H2O
B) CO2
C) SF2
D) ICl3
E) NCl3
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which compound is resonance required to describe the structure adequately?
A) PCl3
B) CO32-
C) HCN
D) NH4+
E) none of these
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for PO43-.
A) square planar
B) octahedral
C) pyramidal
D) tetrahedral
E) none of these
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are in the Lewis structure for NO2-?
A) 18
B) 20
C) 32
D) 16
E) 30
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a nonzero dipole moment?
A) CS2
B) SiF4
C) CH4
D) SO3
E) PBr3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 135
Related Exams