A) I
B) II and IV
C) III and V
D) I and IV
E) V
G) A) and E)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Which of the following processes is responsible for the fact that free radical bromination of methane is slower than free radical chlorination?
A) initiation
B) hydrogen abstraction
C) halogen abstraction
D) termination
E) entropy
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
How many constitutional isomers are possible if propane is dichlorinated? Draw them.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following shows the initiation step of monochlorination of methane? 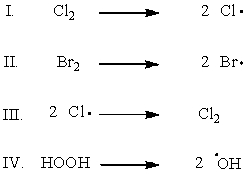
A) I
B) II
C) III
D) IV
E) I and II
G) None of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following are major products of the reaction shown? 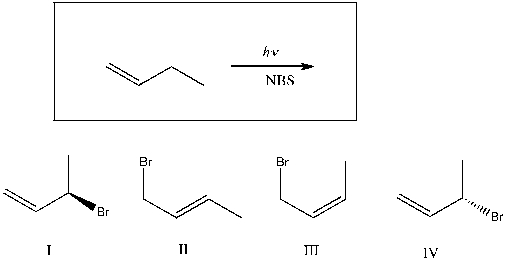
A) I
B) I, II
C) I, IV
D) I, III
E) I, II, III, IV
G) B) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which of the following represents a propagation step in the monochlorination of methylene chloride (CH2Cl2) ?
A) ![]()
B) Cl2
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is expected to function as an antioxidant? 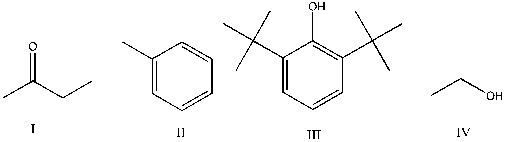
A) I
B) II
C) III
D) IV
E) I, II, III, and IV
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which term most accurately describes the process shown below? 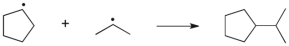
A) hydrogen abstraction
B) halogen abstraction
C) homolytic cleavage
D) coupling
E) elimination
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Upon treatment with NBS and irradiation with UV light, 1-ethyl-4-methylbenzene produces exactly three monobrominated compounds (including stereoisomers). Draw the products of this reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the molecule shown below, determine which of the highlighted C-H bonds (from a to e) is expected to have the lowest bond dissociation energy. 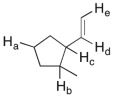
A) C-Ha
B) C-Hb
C) C-Hc
D) C-Hd
E) C-He
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most stable radical?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Free radical chlorination of ethane can produce higher halogenation products (dichlorinated, trichlorinated, etc…) in addition to chloroethane. How could the production of higher halogenated products be minimized?
A) Use an excess of chlorine
B) Use an excess of ethane
C) Use equimolar chlorine and ethane
D) It is not possible to minimize the production of higher halogenated products
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Use correct arrow formalism to draw all of the reasonable resonance structures for the radical shown below. 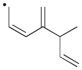
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following radicals in order of decreasing stability (most stable to least stable) . 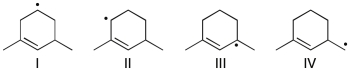
A) IV > I > II > III
B) III > I > II > IV
C) III > II > I > IV
D) III > IV > II > I
E) II > III > I > IV
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Propylene (propene) undergoes free radical polymerization with benzoyl peroxide, but does not produce very long chains. Provide a reasonable explanation for this result.
Correct Answer

verified
There are C-H bonds at allylic positions...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Use correct arrow formalism to show the mechanism of the following radical process: 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds would be expected to be least destructive to the ozone layer?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Propose an efficient synthesis of 3,4-dimethyl-2-pentanol using 2-methyl-2-butene and ethanol as your sources of carbon.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which term best describes the process shown below? 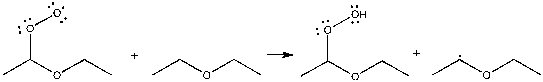
A) neutralization
B) propagation
C) termination
D) initiation
E) elimination
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the major product(s) of the following reaction. Is the product optically active? Explain. 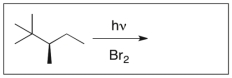
Correct Answer

verified
 The product is not ...
The product is not ...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 1 - 20 of 90
Related Exams