B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the Lewis structure for XeO2F2 which correctly minimizes formal charges.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol for the a lead atom is
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s) .What does the symbol represent?
A) Electron configuration
B) Valence electrons
C) Atomic number
D) Atomic mass
E) Nucleus and core electrons
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule has the largest dipole moment?
A) HF
B) HI
C) HBr
D) HCl
E) All of the molecules have the same dipole moment.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following contains ionic bonding?
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these compounds is most likely to be ionic?
A) GaAs
B) SrBr2
C) NO2
D) CBr4
E) H2O
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these compounds is most likely to be covalent?
A) Rb2S
B) SrCl2
C) CS2
D) CaO
E) MgI2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What types of elements undergo covalent bonding?
A) a metal and a noble gas
B) a nonmetal and a metal
C) two nonmetals
D) two Group 1A elements
E) an actinide
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What formal charge resides on the carbon of CO32-?
A) 0
B) +1
C) +2
D) +3
E) +4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the best Lewis structure for the fulminate ion,CNO-,what is the formal charge on the central nitrogen atom?
A) +2
B) +1
C) 0
D) -1
E) -2
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
Only valence electrons are shown in the Lewis structure held together by covalent bonds.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these compounds is most likely to be covalent?
A) CsOH
B) NF3
C) Sr(NO3) 2
D) CaO
E) LiF
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these choices is the best Lewis structure for ozone,O3?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the compound with the lowest (i.e.,least negative) lattice energy.
A) CsBr(s)
B) NaCl(s)
C) SrO(s)
D) CaO(s)
E) KBr(s)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The total number of bonding electrons in a molecule of formaldehyde (H2CO) is
A) 3.
B) 4.
C) 6.
D) 8.
E) 18.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many dots does the Lewis dot symbol for oxygen have around it?
A) 4
B) 2
C) 6
D) 3
E) 7
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formal charge on Cl in the structure shown for the perchlorate ion is _____. 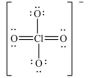
A) -2
B) -1
C) 0
D) +1
E) +2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of resonance structures for the sulfur dioxide molecule that satisfy the octet rule is
A) 1.
B) 2.
C) 3.
D) 4.
E) none of these
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
A single bond between two atoms represents ________ pair(s)of electrons.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 95
Related Exams